The cortex and brain tumors
Multiple childhood brain tumors, like cortical gliomas, arise from the cortex, the outer layer of the largest part of the brain and the brain’s most expanded structure. Currently, around six in ten children are still alive five years after they were diagnosed with a malignant tumor of the central nervous system. Research into a better understanding of how brain tumors arise and develop could help researchers in finding possible targets for treatment.
To study the development of the brain and brain tumors, scientists use organoids: 3D mini-organs or mini-tumors grown in the lab. In a new study, scientists at the Princess Máxima Center and the Hubrecht Institute created a new cortex organoid that better represents the human brain.
More accurate models
The new study led by dr. Benedetta Artegiani, research group leader at the Máxima Center and dr. Delilah Hendriks, Oncode-researcher at the Hubrecht Institute and affiliated group leader at the Máxima Center, published today in Nature Communications. The new cortex organoid that they generated more closely represents the human brain in multiple aspects: their shape, their architectural organization and several properties of their cells.
Recapitulate aspects of the developing human brain
The formation of the human brain starts with a single structure, called the neural tube, which is composed of a specific tissue, called the neuroepithelium. The cells in this structure are then slowly ‘instructed’ to generate all the different cells that are present in the various parts of the brain.
Artegiani says: ‘The brain organoids that already existed generally consist of several developmental structures that act like ‘independent’ small developing brains within one organoid.. Researchers have tried for a long time to mitigate the formation of these multiple structures. To diminish variability, increase reproducibility, better recreate the brain’s different cellular identities, and ultimately to recapitulate brain development more closely. We have succeeded in this effort, by creating the current organoids that are composed of a self-organizing, single developing neuroepithelium.’
Anna Pagliaro, PhD student and first author of the study: ’We tried to mimic, in a dish, gradients of molecules that are present during brain development over time. This resulted in mini-brains that are very different in shape and structure than we used to work with. These organoids overall better resemble the early stages of brain development. It is quite impressive to see how much the shape of these organoids can influence all the different cells that form them, both in terms of their shape, architecture but also identity.’
Mimicking mechanisms of the human brain
The researchers named the new mini-brains Expanded Neuroepithelium Organoids (ENOs) for the developmental tissue used to grow them. To generate them, they made a small but important change. Hendriks: ’Cells in the brain are instructed to acquire their identity through molecules that act slowly in time, the so-called temporal gradients. This is exactly what we tried to mimic. To our surprise, it was enough to just provide one of the molecules (TGF-b) slowly step-by-step to generate brain organoids. This tiny change had an enormous impact and allowed us to generate organoids with a shape and an identity more similar to the human brain.’
The next steps
Pediatric brain tumors may derive from unusual or mistaken developmental process and their study could benefit from these new models that better recapitulate early embryonic developmental mechanisms. The signaling molecule that the researchers used to make the organoids is often altered in childhood brain tumors, also suggesting that the onset of cancer in young children could be linked with changes in brain development. Now that the researchers understand that temporal gradients are of great importance to generate more accurate organoids, this study paves the way to develop brain organoids more and more similar to the developing human brain.
Artegiani: ‘We can use these novel organoids as a basis to model pediatric brain tumors, and study more in depth the role of TFG-b signaling in this process. Our research marks an essential step to create proper models to study pediatric brain cancer. If such a small change of a signaling molecule has such a great impact on brain organoid models, we can only start to imagine which effects small alterations during development can have for how pediatric brain tumors can develop.’
This study was partially funded by a NWO open competition M-grant.
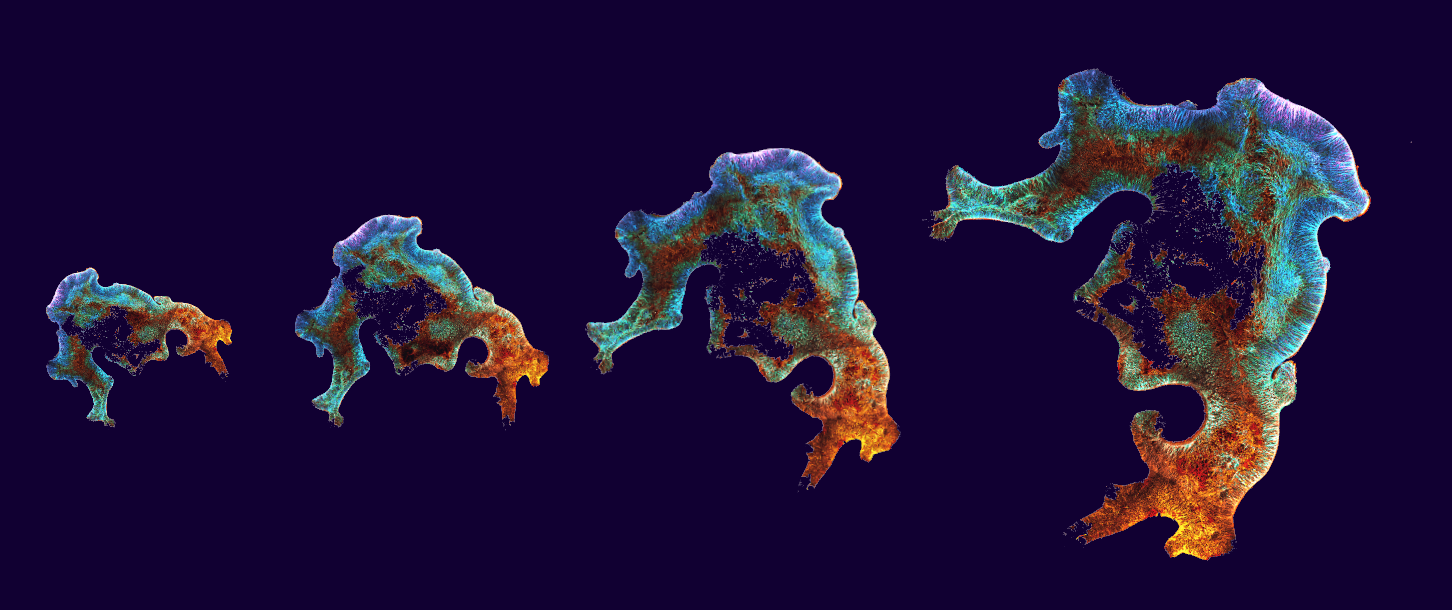
Image of novel brain organoid model showing its characteristic convoluted shaped and extended neuroepithelium. The neurons present in the organoid are shown in different colors depending on their location within the tissue.
Credits: Benedetta Artegiani, Delilah Hendriks and Anna Pagliaro

