Group leader: Dr. Weng Chuan Peng
Liver cancer in children
Each year, 8 to 10 children in the Netherlands are diagnosed with liver cancer, a number that is rising partly due to an increase in premature births. Among these young patients, the most prevalent tumor types are hepatoblastoma and hepatocellular carcinoma. Hepatoblastoma presents in various histological subtypes, including the more differentiated fetal subtype and the more undifferentiated embryonal subtype. In contrast, hepatocellular carcinomas display distinct pathological and biological characteristics, compared to hepatoblastoma. Hepatocellular carcinoma in adolescence and young adulthood often arises due to an underlying metabolic liver disease. These rare hereditary conditions, frequently caused by single gene defects, can lead to severe liver dysfunction. While liver transplantation is often the only curative option, it presents significant challenges including shortage of donor organs and the risk for rejection, underscoring the need for innovative treatment modalities. The rarity of childhood liver cancers and metabolic liver diseases pose significant challenges for research, highlighting the need for dedicated studies on these specific patient populations.

Liver Tumor Organoids
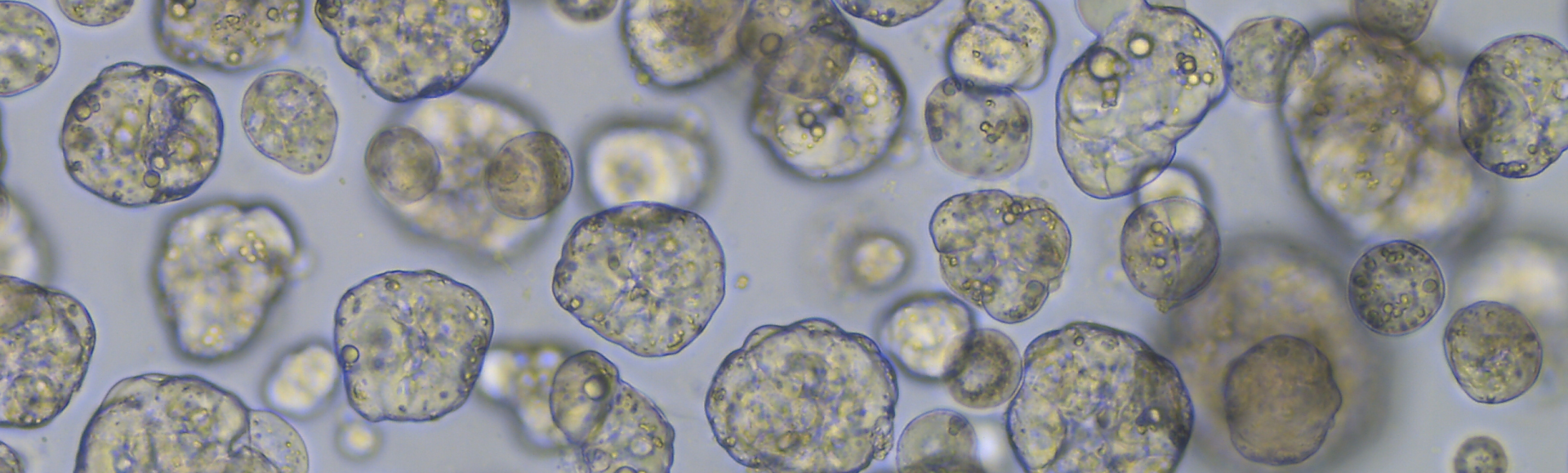
Given the rarity of childhood liver tumors, the patient-derived tumor organoid models established by the Peng group are an invaluable resource for preclinical research. We were the first to establish an extensive cohort of patient-derived tumor organoid models from various disease stages of hepatoblastoma. These lab grown tumor cells are used for various studies, such as the modeling efficacy of engineered T cells, drug screening, gene editing and testing RNA-therapeutics. In addition, we contributed to the generation of organoids models for pediatric liver cancer as part of the ITCC-P4 consortium and the Dutch Oncode Accelerator foundation.
Primary Hepatocyte Organoids
Mature hepatocytes, including diploid and polyploid cells, can be induced to proliferate, without the need for stem cells. In 2018, we were the first to demonstrate that mouse hepatocytes, the major cell type in the liver, can be propagated indefinitely in 3D organoid cultures, by employing factors that are associated with tissue repair. Of particular significance is that hepatocyte organoids can efficiently engraft into the injured livers of mice and restore liver function. The ability to generate large number of stem cells is the first essential step towards making cell replacement therapy possible in patients with liver disease, potentially addressing the global challenge of organ donor shortages. We are also actively engaged in the development of more advanced organoid models.
Single-cell, spatial transcriptomics, high-plex imaging
We are dedicated to uncovering the intricacies of liver pathologies through new technology. One of our main goals is to decipher the molecular heterogeneity of various pediatric tumor subtypes, pinpoint critical signaling pathways, and explore the tumor immune microenvironment. To achieve these objectives, we harness multiomic approaches, including single-cell analysis of the transcriptome and epigenome, spatial transcriptomics, high-plex imaging methods and label-free mass spectrometry-based proteomics.
Development of therapeutics
We leverage patient-derived tumor organoid models and insights from omics to develop new therapeutics. We aim to find tumor-specific therapeutic strategies to treat liver cancer patients, exposing fewer children to high-doses of chemotherapy, and ultimately curing more children without compromising their quality of life. Potential novel treatment avenues for these patients include immunotherapies, antibody therapies, and small molecule inhibitors. For metabolic liver disease patients, potential treatments can be searched in gene editing, RNA-therapeutics and cell replacement therapies.
We collaborate closely within the Princess Máxima Center Comprehensive Childhood Cancer Center (M4C) Liver Tumor Group, where Dr. Peng serves as the research chair and Dr. Zsiros, a pediatric oncologist, serves as the clinical chair. This structure fosters strong interactions between our preclinical research team and clinicians from various specializations, enhancing the translational impact of our work.
Our team is actively involved in the International Childhood Liver Tumor Study Group (SIOPEL) and has extensive experience leading international multi-center clinical trials and consortia. We play key roles in both the Pediatric Hepatic International Tumor Trial (PHITT) and the Children’s Liver Tumor European Research Network (CHILTERN) consortium, contributing to advancements in pediatric liver cancer research and treatment.
- Integrated multiomic and drug profiling of pediatric liver tumor organoids to investigate tumor biology and to identify novel therapeutic strategies - KiKa Research Grant (2024-2026). News Article
- Oncode Accelerator Foundation, Dutch National Growth Fund (2024-2026).
- KWF Research Project (2024-2027), with Dr. Dennis Beringer, Prof. Jūrgen Kuball. News Article
- STOmics Grant Program, Spatial Temporal Omics Consortium (2023-2024). Consortium website
- ITCC-P4 Organoid Mirror Project (2023-2024), with Molenaar group.
- Strategic Program Cancer Grant, UMC Utrecht (2022-2023), with Dr. Yvonne Vercoulen, Dr. Daniëlle Krijgsman.
- Twinning program KiTZ . Toward precision medicines for liver tumors – with dr. Zsiros, dr. Ina Oehme(KiTZ) (2022-2023)
- Donations are regularly received through the Princess Máxima Center Foundation. With gratitude, the Peng group spends these donations on the generation and characterization of patient-derived tumor organoid models. An important donor is ‘Kus van Kiki’.
Kluiver TA#, Lu Y#, Schubert SA, Kraaier LJ, Ringnalda F, Lijnzaad P, DeMartino J, Megchelenbrink WL, Amo-Addae V, Eising S, de Faria FW, Münter DJ, van de Wetering M, Kerl K, Duiker E, van den Heuvel M, de Meijer VE, de Kleine RH, de Krijger RR, Molenaar JJ, Margaritis T, Stunnenberg H, Zsiros J, Clevers H, Peng WC*. Divergent WNT Signaling and Drug Sensitivity Profiles within Hepatoblastoma Tumors and Organoids. Nat Commun.2024; 15, 8576. Read here.
Krijgsman D#, Kraaier LJ#, Verdonschot M, Schubert SA, Duiker E, de Kleine R, de Meijer V, de Krijger RR, Zsiros J, Leusen J, Peng WC*, Vercoulen Y*. Hepatoblastoma exhibits a predominantly myeloid immune landscape and reveals opportunities for macrophage targeted immunotherapy. 2023. BioRxiv. Read here.
Kluiver TA, Kraaier LJ, Peng WC*. Long-Term Expansion of Murine Primary Hepatocyte Organoids. Methods Mol Biol. 2022;2544:1-13. Read here.
Peng WC*, Kraaier LJ, Kluiver TA. Hepatocyte organoids and cell transplantation: What the future holds. Exp Mol Med. 2021;53(10):1512-1528. Read here.
Tabula Muris Consortium. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020 Jul;583(7817):590-595. Read here.
Peng WC*, Logan CY, Fish M, Anbarchian T, Aguisanda F, Alvarez A, Wu P, Jin Y, Zhu J, Li B, Grompe M, Wang B, Nusse R*. Inflammatory cytokine TNFa promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 2018. 175, 607–1619.e1615. (*corresponding authors, F1000 recommendations) Read here.
Tabula Muris Consortium. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018 Oct;562(7727):367-372. Read here.








