Group leader: Prof.dr. Jan Hoeijmakers
Damage to our genome occurs continuously in every cell at a massive scale. Time- and exposure-dependent accumulating DNA damage leads to mutations that can initiate and drive carcinogenesis or -as we discovered- physically hamper transcription, triggering functional decline, cellular senescence and cell death causing aging. Our main research focus is the interplay between DNA damage accumulation and repair in cancer (therapy) and (accelerated) aging and neurodegeneration. By studying molecular mechanisms up to patients, we intend to obtain an integral understanding and derive rational-based effective strategies, including nutritional and pharmacological interventions which promote overall healthy aging and reduce severe long-term side effects and improve quality of life in children cured from cancer.
DNA Damage and Repair
The research of the Hoeijmakers group focusses on DNA damage accumulation and repair and its consequences for cancer and aging: the main healthcare problems in developed societies. The Hoeijmakers team cloned the first human DNA repair gene, ERCC1, followed by many more, discovered the very strong evolutionary conservation of DNA repair and an unexpected link with basal transcription. This elucidated the molecular basis of the cancer-prone repair disorder xeroderma pigmentosum (XP) and the -till then enigmatic- neurodevelopmental repair conditions, Cockayne syndrome (CS) and trichothiodystrophy (TTD). His team identified the XPC protein as the key DNA damage recognition factor, the 10-subunit TFIIH complex as local unwinding component and the ERCC1/XPF endonuclease together with XPG involved in damage excision. He was the first to synthesize the outline of the nucleotide excision repair (NER) reaction mechanism. In addition, the Hoeijmakers laboratory pioneered the in vivo analysis of the dynamics of DNA repair by fluorescent tagging in living cells and even living mice in combination with local DNA damage induction. This opened a new field of DNA repair research that explores repair in the most relevant context: the living cell and intact mammal. Simultaneously, his team embarked upon the systematic generation of mouse repair mutants, to bridge the gap between cells and patients. The mouse mutants turned out to be extremely informative: they mimicked the corresponding human syndromes to an exceptional degree and enabled detailed insight into the complex etiology of human repair diseases, including the initially highly controversial identification of many features of accelerated, but fully bona fide aging. This disclosed a balance between cancer and aging and the link of both with DNA damage.
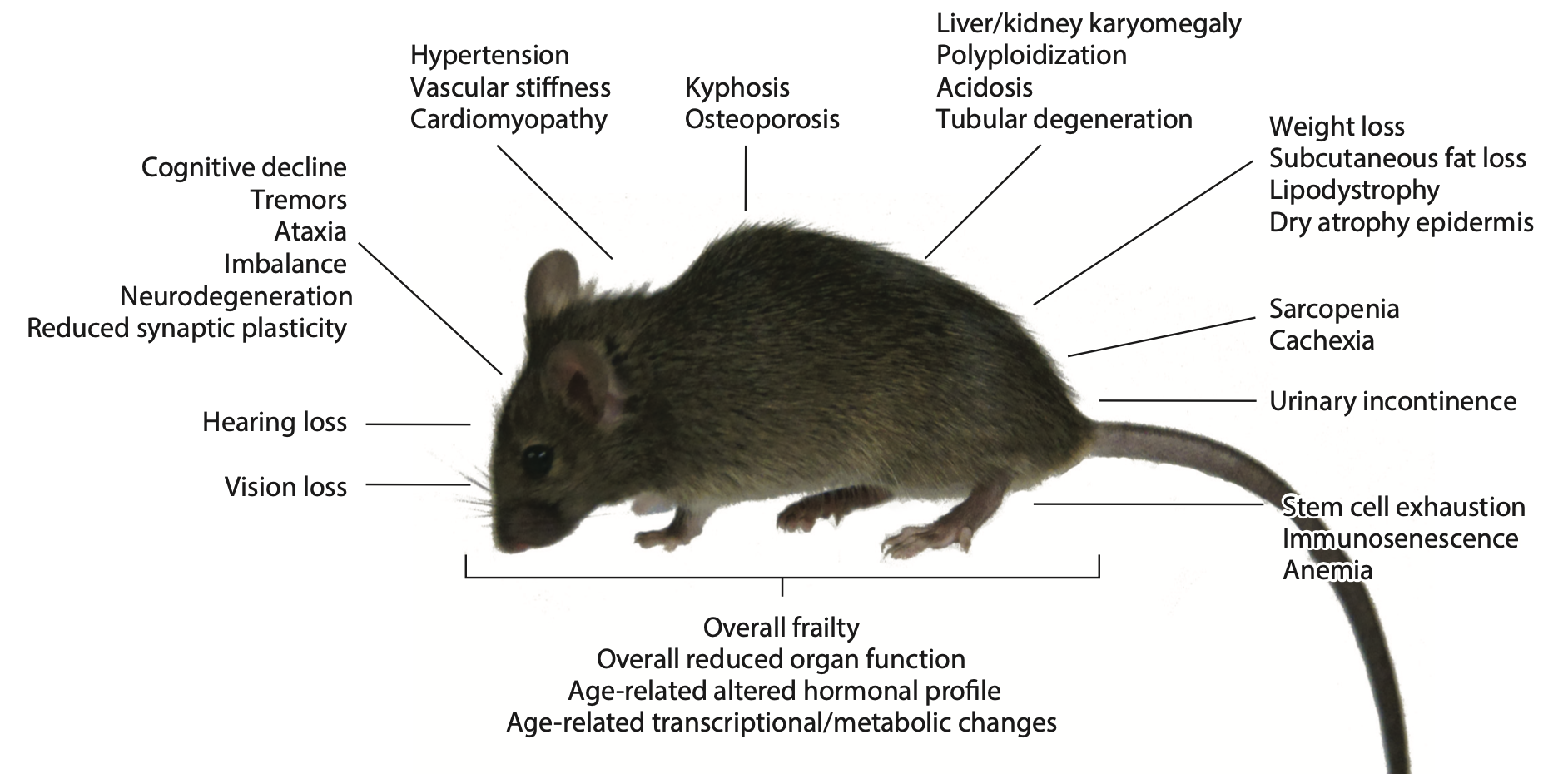
Aging features and age-related pathology in Ercc1 mutant mice.
Connection with Cancer and Aging
His team identified which repair processes primarily protect from cancer and which from accelerated aging and succeeded in getting grip on the aging process in mice by modulating DNA repair and, surprisingly, by nutritional interventions. The acceleration of specific aging features strictly correlate with the severity of defects in specific repair pathways. The spectrum of accelerated aging symptoms (which organs age fast) is determined by the type of repair defect (which pathway is affected). E.g., transcription-coupled repair, which removes DNA lesions that physically stall transcription, primarily protects post-mitotic tissues such as the neuronal system from accelerated aging, cross-link repair the proliferative organs such as the hematopoietic system. Conditional repair mutants allowed targeting accelerated aging to any organ, tissue or stage of development, for instance, mice in which only the cerebellum or heart exhibit dramatic accelerated aging. The hypomorphic Ercc1Δ/-mutant, affected in 4 repair pathways, displays the most wide-spread premature aging phenotypes documented to date for any mammal: progressive neurodegeneration (dementia, ataxia, loss of hearing, vision, neuronal plasticity, etc.), osteoporosis, cardiovascular, hematological and immunological aging, thymic involution, cachexia, sarcopenia, early infertility, liver, kidney aging etc., accompanied by progressive behavioral-physiological-hormonal alterations, loss of stem cells, increased cellular senescence, overall frailty and gene expression patterns alike natural aging. Importantly, this mutant is a superior model for Alzheimer and other neurodegenerative diseases addressing a tremendous unmet medical need, consistent with the notion that aging is the most important risk factor for proteinopathies.
Counteracting Damage by Nutrition
Rapid accumulation of unrepaired DNA damage in these mice may cause cancer and/or premature (cell) death and senescence, but triggers also an anti-aging, anti-cancer response likely in an attempt to extend their short lifespan. This ‘survival’ response suppresses growth and enhances maintenance and defense systems (anti-oxidant defenses, stress resistance, immunological and metabolic parameters) and -interestingly- resembles the anti-aging longevity response induced by dietary restriction (DR). Strikingly, subjecting progeroid, dwarf mutants to actual DR resulted in the largest lifespan increase recorded in mammals: 30% DR tripled median and maximal remaining lifespan, and drastically retarded all aspects of accelerated aging investigated, but most impressively neurodegeneration. DR animals retained 50% more neurons, maintained full motoric function, and even lost tremors not only arresting neuronal decline, but even improving neurofunctioning. Repair-deficient progeroid Xpg-/- mice responded similarly to DR, extending this observation beyond Ercc1. The DR response in Ercc1Δ/- animals resembled DR in wt. Importantly, ad libitum Ercc1Δ/- liver showed a progressive dramatic genome-wide decline of overall transcription, preferentially of long genes. This lowered and imbalanced transcriptional output was subsequently also discovered in normal aging in numerous post-mitotic tissues in many species including humans, and appeared even present in C.elegans, demonstrating its universal occurrence and stressing the value of progeroid repair-deficient mutants for normal aging. Moreover, this phenomenon of transcription stress was shown to be the direct consequence of DNA damage blocking elongating RNA polymerase affecting genes proportional to length.
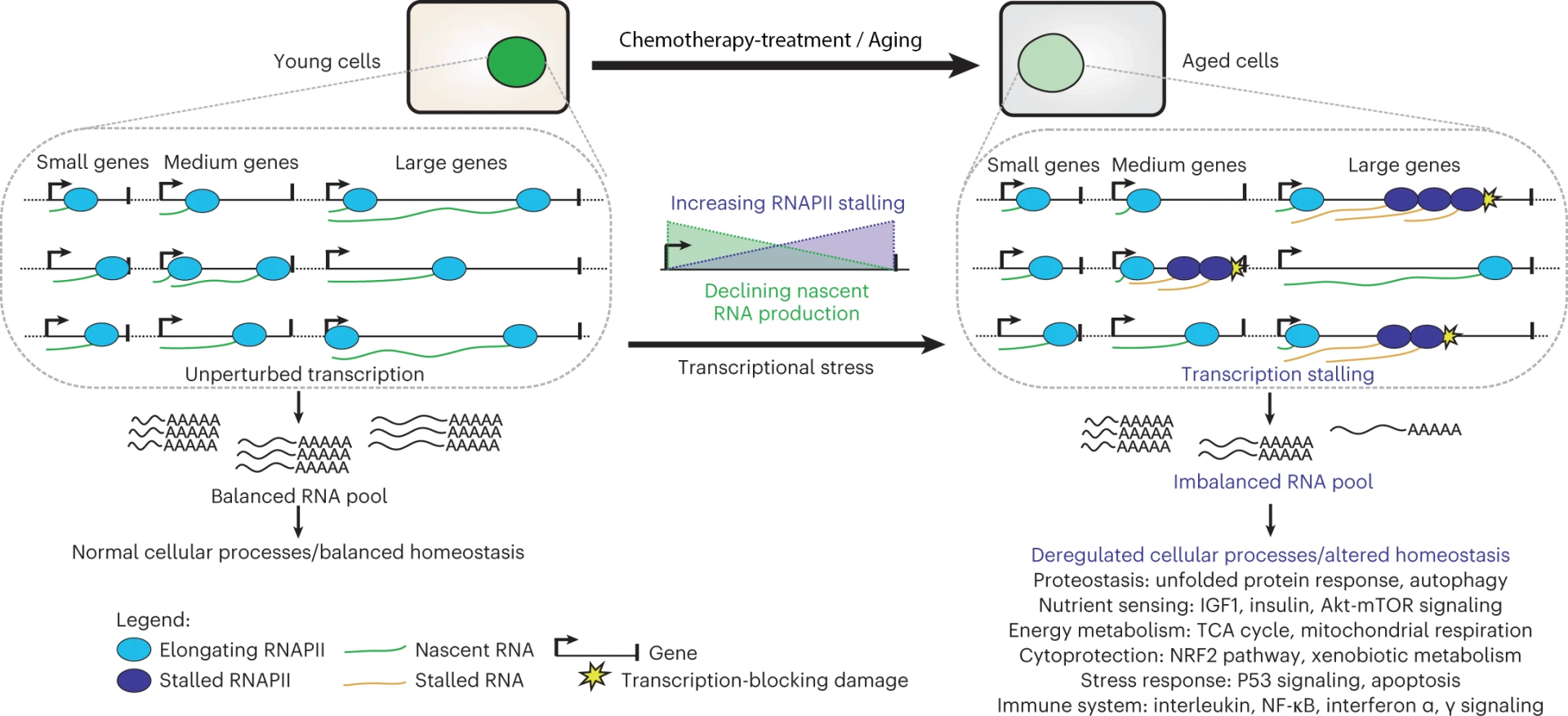
As transcription is essential for all cellular processes, DNA-damage-driven transcription stress impacts numerous cellular processes, found to change with aging, revealing how accumulation of DNA damage causes aging in most of the soma. DR largely prevented this decline of transcriptional output, indicating that DR reduces DNA damage levels and prolongs genome function and revealing how DR delays aging. These findings strengthen the link between DNA damage and aging, provide insight into the molecular mechanism underlying DR, establish Ercc1Δ/- mice as powerful model for identifying interventions to promote healthy aging, reveal untapped potential for reducing endogenous damage, and suggest a counterintuitive DR-like therapy for human progeroid genome instability syndromes and DR-like interventions for preventing neurodegenerative diseases. Indeed, reducing calorie intake in CS and TTD children, which normally get extra nutrition as they are severely growth-retarded, induced dramatic improvements, most spectacularly in neurological performance, and most likely extends life expectancy, constituting the first therapeutic intervention for these dramatic syndromes. This stresses the clinical importance and the validity of the findings in the corresponding mouse mutants.
Relevance for Pediatric Oncology
These findings are particularly relevant in the context of pediatric oncology, where chemo/radiotherapy given to children as treatment against their cancer, also results in DNA damage accumulation is other organs and tissues, thereby contributing to the development of direct and/or late-life side effects and (segmental) accelerated aging. By means of nutritional preconditioning, DR and/or fasting regimens could be implemented prior to treatment, to have the remarkably powerful protective ‘survival response’ activated at the start of therapy. The boosted defense and resilience mechanisms, reduce DNA damage, delay aging and protect from surgery-associated ischemia/reperfusion-injury and chemo/radiotherapy. We study these concepts and underlying mechanisms using cell systems, organoids, organotypic tissue slices, mouse models and in clinical studies in patients with the aim to improve quality of life of all (ex-)cancer patients.
In summary, this pioneering work places DNA damage at the basis of cancer and aging, highlights the flexible nature of aging and establishes the repair mutants as valid tools for identification of life- and healthspan-extending pharmaceutical and nutraceutical interventions in mammals. This opens new avenues for prevention or treatment of aging-related diseases, including cancer.

- The use of organotypic tissue slices for assessing prevention against treatment-induced side-effects via nutritional preconditioning interventions – CoFund Butterfly (2023 – 2027) - News article
- Oncode Institute phase 2 (2023 – 2027) - News article
- MODEM: Mechanisms Of DEMentia (with Guus Smit, Elly Hol, Wiep Scheper, Bart Eggen, Rik van der Kant, Daniel van den Hove and others) - ZonMW Multidisciplinaire Consortia van het Onderzoeksprogramma Dementie (2023 – 2027) - News article
- CureQ: Predict, Delay & Cure polyglutamine (Q) caused neurodegeneration (with Eric Reits, Ineke Bolt, Rob Haselberg, Jurre Hageman, Jurjen Helmus, Harm Kampinga, Monique Mulder, Mayke Oosterloo, Willeke van Roon, Bart van Warrenburg, and Tsjerk Wassenaar) – NWO NWA-ORC (2022 – 2027)
- TC-NER: Transcription stress Counteracted by Nutritional interventions of Exceptional importance for rare DNA Repair disorders (with Vincent Laugel, Pier G. Mastroberardino, Umut Altunoglu, Akos Gyenis, Wim Vermeulen, and patient organization Amy and Friends) – European Joint Programme Rare Diseases (2021 – 2024)
- RedioDeal Foodvalley – Spoor 2 Gezonde voiding: van prille start tot oude dag (with Wim Tissing, Anne May, Ellen Kampman and others) – Rijksoverheid, Provincie Gelderland en Provincie Utrecht (2020 – 2024)
- Investigating the role of DNA repair mechanisms in the development of age-related podocyte malfunction and its consequence on key organs (with Tobias Huber, Oliver Kretz and Ketan Patel) – DFG (2020 – 2024)
- Understanding the pathogenicity of mutant Huntingtin conformations and their modulation by the chaperonin TRiC/CCT - Campagneteam Huntington (2020 – 2023)
- Nutritional prevention of dementia – ZonMW Memorabel (2018 – 2023)
- Muscle Ageing: Identification of Building Blocks, Biomarkers, and Strategies to Sustain Health – NWO Building Blocks of Life (2018 – 2022)
- Oncode Equipment Fund for Seahorse Cell Metabolism Technology - Measuring functional metabolism in pediatric oncology
Twinning program KiTZ
- Nutritional preconditioning to enhance chemotherapy efficacy and reduce toxicity in pediatric cancers – with Marcel Kool (KiTZ) (2021-2023)
Selection of Awards
- 2022: Oncode Investigator, renewal selection for five years
- 2021: Official recognition of the Erasmus MC clinical expertise center for Rare Genome Instability Disorders, a multi-disciplinary clinic for children with DNA repair syndromes, initiated by Jan Hoeijmakers
- 2020: Ammodo Research Team Award” to ‘Guardians and Caretakers of the Genome’ together with other members of the Erasmus Dept. Mol. Genet.
- 2019: “EMGS Award” of the Environmental Mutagenesis and Genetics Soc. (Washington DC)
- 2017: International Olav Thon Foundation personal Award
- 2016: NVHG Galjaard Prize of the Netherlands Society of Human Genetics
- 2016: Professor International Faculty, Cologne University (Cologne, Guest Professor)
- 2016: Selected for the Nobel-Forum lecture at the Karolinska Institute
- 2015: ERC PoC grant DEMENTIA European Research Council
- 2014: Consulted by the Nobel Committee for the Nobel Prize in Chemistry for DNA repair (2014-2015)
- 2013: Royal distinction Knight in the Order of the Dutch Lion for important scientific achievements in the area of cancer and aging research (2013).
- 2012: Mendel Medal on the occasion of the 190th anniversary of Mendel’s birth
- 2011: Academy Professor of the Royal Academy of Sciences of The Netherlands (KNAW), First Academy Professor new style in the broad domain of Beta sciences
- 2008: Seneca Medaille des Industrie-Clubs für Altensforschung Prize, for pioneering research on the molecular basis of aging (First awardee)
- 2001: ‘Josephine Nefkens Prize’ for cancer research
- 2000: Elected member of KNAW (section ‘Medicine’, dept. ‘Physics’)
- 2000: ‘EC-Descartes’ Award for European collaboration on DNA repair
- 1999: ‘Spinoza’ Prize, most recognized prize of the Dutch Science Organization
- 1995: The very prestigious 'Louis Jeantet' Prize for Medical Research in Europe for the entire work on DNA repair (Geneva, 1995)
- 1986: 'Snoo van t' Hoogerhuys' Prize (isolation of the first human DNA repair gene)
- 1983: 'Harold Quintus Bosz' Prize (for the discovery of the molecular mechanism of antigenic variation in trypanosomes, PhD thesis)
de Boer J., Andressoo J.O., de Wit J., Huijmans J., Beems R.B., van Steeg H., Weeda G., van der Horst G.T.J., van Leeuwen W., Themmen A.P.N., Meradji M. and Hoeijmakers J.H.J. Premature aging in mice deficient in DNA repair and transcription. (2002) Science (research article), 296:1276-1279. (see also Comments in Science, 296, 1250-1251, and in DNA Repair 2, 437-439). PubMed PMID: 11950998
Niedernhofer L.J., Garinis G.A., Raams A., Lalai S.A., Robinson R.A., Appeldoorn E., Odijk H., Oostendorp R., Ahmad A., van Leeuwen W., Theil A., Vermeulen W., van der Horst G.T., Meinecke P., Kleijer W., Vijg J., Jaspers N.G.J., Hoeijmakers J.H.J. A new progeriod syndrome reveals that genotoxic stress suppresses the somatotroph axis. (2006) Nature (research article) 444:1038-1043. (see also accompanying ‘News and Views’ Nature by Kirkwood). PubMed PMID: 17183314
Hoeijmakers J.H.J. DNA damage, aging, and cancer. (2009) N Engl J Med 361:1475-85. PubMed PMID: 19812404
Marteijn J.A., Lans H., Vermeulen W. and Hoeijmakers J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. (2014) Nature Rev Mol Cell Biol 15:465-481. PubMed PMID: 24954209
Vermeij W.P., Dollé M.E.T., Reiling E., Jaarsma D., Payan-Gomez C, Bombardieri C.R., Wu H., Roks A.J.M., Botter S.M., van der Eerden B.C., Youssef S.A., Kuiper R.V., Nagarajah B., van Oostrom C.T., Brandt R.M.C., Barnhoorn S., Imholz S., Pennings J.L.A., de Bruin A., Gyenis Á., Pothof J, Vijg J, van Steeg H., and Hoeijmakers J.H.J. Restricted diet delays accelerated aging and genomic stress in DNA repair deficient mice. (2016) Nature 537:427-431 (see also accompanying Nature ‘News and Views’ of Oshima and Martin, p316-317). PubMed PMID: 27556946
Schumacher B., Pothof J., Vijg J., Hoeijmakers J.H.J. The central role of DNA damage in the ageing process. (2021) Nature 592:695-703. Review. doi: 10.1038/s41586-021-03307-7. PubMed PMID: 33911272
van den Boogaard W.M.C., van den Heuvel-Eibrink M.M., Hoeijmakers J.H.J., Vermeij W.P. Nutritional Preconditioning in Cancer Treatment in Relation to DNA Damage and Aging. (2021) Annu Rev Cancer Biol 5:161-179 PubMed PMID: 35474917
van den Boogaard W.M.C., Komninos D.S.J., Vermeij W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal (2022) Cancers (Basel) 14:627 (Cancers 2022 Best Paper Award). PubMed PMID: 35158895
Gyenis A., Chang J., Demmers J.J.P.G., Bruens S.T., Barnhoorn S., Brandt R.M.C., Baar M.P., Raseta M., Derks K.W.J., Hoeijmakers J.H.J., Pothof J. Genome-wide RNA polymerase stalling shapes the transcriptome during aging (2023) Nat Genet 55:268-279. PubMed PMID: 36658433
- Our fundamental research has, in close consultation with parents, parent family organizations (Amy & Friends, UK and NL, Em’ma Vie, FR), dieticians, clinicians, and researchers, led to a complete change of nutritional guidelines for the progeriod DNA repair syndrome trichothiodystrophy (TTD) - Nutritional Guidelines for Trichothiodystrophy
- Fasting Intervention for children with Unilateral Renal Tumours to reduce Toxicity (FIURTT) - FIURTT-Study - Máxima
- Fasting before live kidney donation, effect on donor wellbeing and postoperative recovery (FAST) - FAST-Study - EMC/UMCG
- KETOgenic diet therapy in patients with HEPatocellular adenoma (KetoHeppy) - KetoHep(py) - EMC

















