Group leader: Prof. dr. Jan Molenaar
Targeted drug development
Lead: Marlinde van den Boogaard
A highly efficient pipeline covering the full spectrum of targeted drug development is currently functional. The process starts with the validation of targets using high throughput analysis as Affymetrix mRNA profiling, RNAseq, Whole Genome Sequencing and single cell analysis. We are now building a dataset of over 500 neuroblastoma tumors for which WGS and RNA profiling data is available. We are performing integrative analysis on these samples that will allow identification of new driving events and relate genomic events to clinical subgroups within neuroblastoma. Potential new target genes are subsequently validated in cell line systems using a variety of molecular genetic manipulation techniques. The most promising target genes are then further validated using small molecule inhibitors in vitro and in vivo. Several new models are developed to consolidate this process. Tumor organoids are used and new in vivo xenograft and transgenic models are implemented. The current studies are in various stages of development and targets are MDM2, CDK4/6, CDKN2A, the MEK pathway, BCL2/MCL1, CHK1, ATRX and MTH1 in neuroblastoma with the CtoA mutator phenotype.
Immune therapy
Lead: Judith Wienke
The aim of this project is to identify novel targets for immunotherapeutic interventions in neuroblastoma and to develop and investigate innovative (combinations of) immunotherapeutic interventions for clinical applicability. We are investigating the immune environment of neuroblastoma, and possibly other tumors, starting with single cell RNA sequencing and isolation of tumor-infiltrating immune cells to identify novel targets for immunotherapy. Target identification will be aimed at both undermining tumor immune evasion and increasing the anti-tumor effectivity of immune cells. After target validation, different innovative strategies and combinations of immunotherapeutic interventions will be tested; first in vitro on tumor cell lines and organoids, and later in vivo in murine models. Ultimately, effective interventions will be introduced into the clinical trial phase.
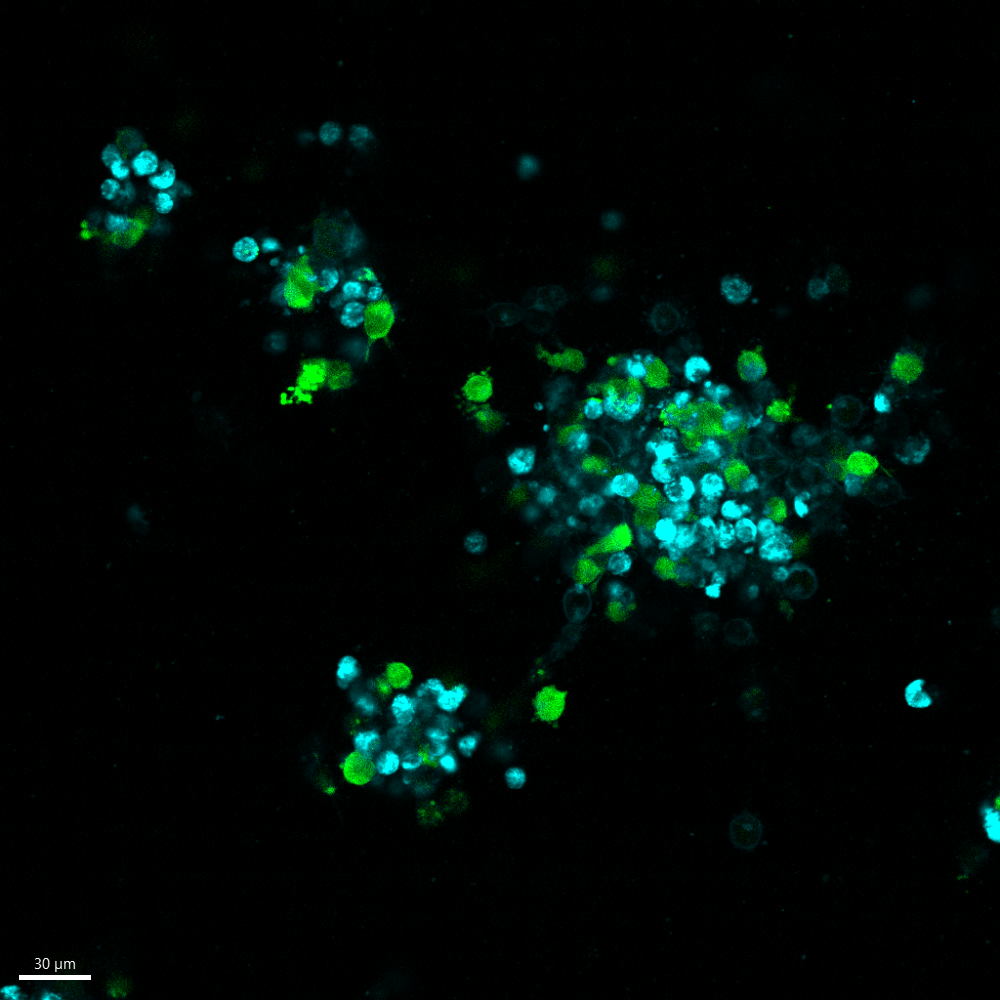
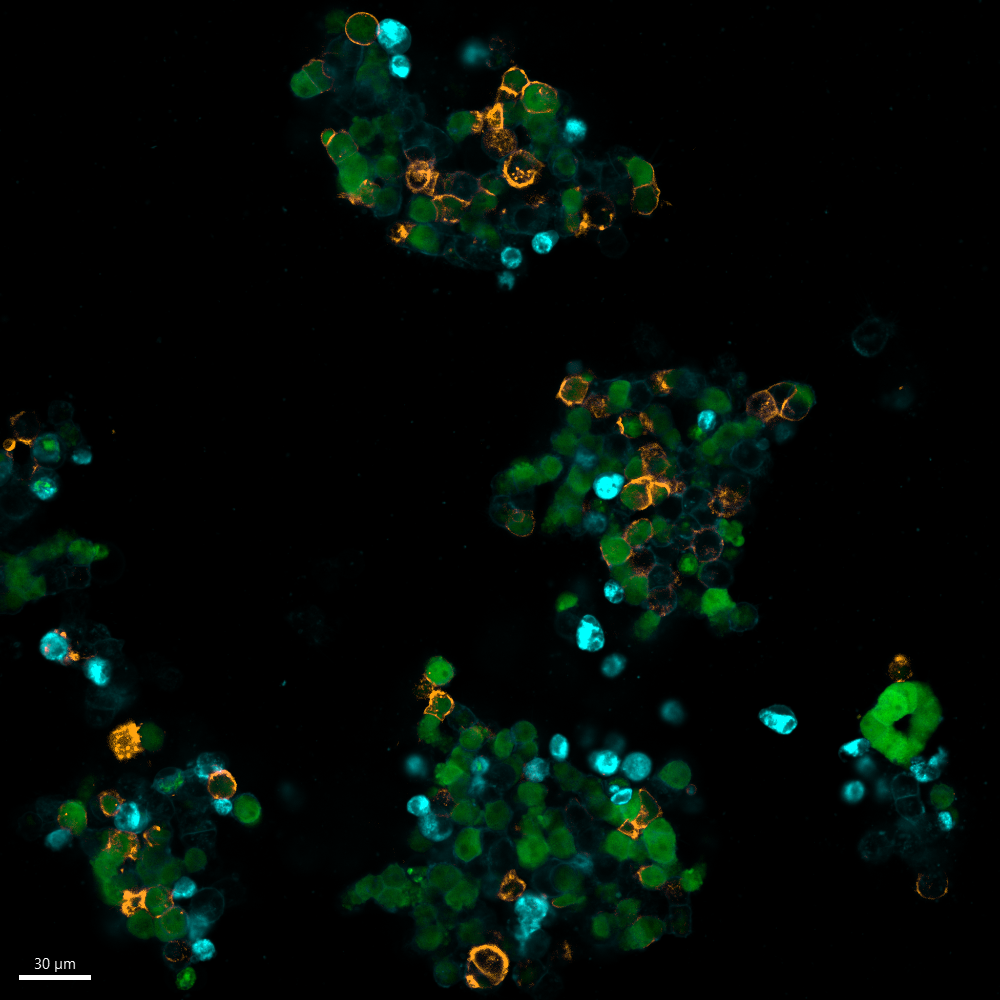
In this figure, we illustrate the binding of Dinutuximab (anti-GD2) to two of our Luc-GFP transfected organoid models: 691T (left) and 691B (right). The 691T organoids, derived from the tumour, lacks GD2 expression, while the 691B organoids, derived from the bone marrow of 691T, expresses GD2 on its cell surface. The GFP construct is shown in green, and CellBrite®, a cytoplasmic membrane marker, is shown in blue. The fluorescently labelled Dinutuximab, depicted in orange, binds to the 691B organoid (right), as it expresses GD2 on the cell surface. Since the 691T organoid (left) does not express GD2, Dinutuximab is not able to bind to these cells.
The precision medicine program iTHER
Lead: Karin Langenberg
The goal of the iTHER (individualized THERapy) program is to realize personalized treatment for children with relapsed or refractory, incurable cancer. This involves 150 patients per year. The program is a METC approved clinical registry trial which is performed in collaboration with the groups of Michel Zwaan, Patrick Kemmeren and the diagnostic lab from Bas Tops. Molecular characterization includes Exome Sequencing, RNAseq, and methylation pattern analysis using the Máxima center's diagnostics pipeline. Data is stored at the core facility of the Máxima center and analyzed using the R2 bioinformatics platform. We have obtained an NWO precision medicine grant to further develop the iTHER program, which involves clinical implementation of this program in the Máxima, connecting the program to targeted therapies and expanding our profiling with in vitro compound testing on patient derived tumor organoids.
Compound screening and chemistry
Lead: Selma Eising
The aim of this project is to identify and validate new treatment options in pediatric cancer using the high throughput screening facility. The two major objectives are: 1) The coordination of compound screening facility which has become available in 2019. It contains a state of the art BeckmanCoulter robotics set-up including an Echo550 compound dispenser system. The facility runs many screens for different research groups in the Máxima center. 2) The incorporation of in vitro model systems to validate potential interventions, identify new interventions and identify potential combination treatment options for patients without curative options, as a part of the iTHER precision medicine program.
Furthermore, a new line of research is set up that aims to use a novel class of drugs called PROteolysis Targeting Chimerase (PROTACs) for the treatment of neuroblastoma. In the Molenaar group, we have identified frequent chromosomal rearrangement and genomic aberrations that are driving neuroblastoma. Two of these specific oncogenic drivers are defined as ‘un-targetable’, because they cannot be targeted using existing drugs. In collaboration with the Utrecht Institute of Pharmaceutical Sciences, we aim to selectively degrade the tumor-target proteins using PROTACs.
- Making CDK inhibitors work for pediatric cancer – KWF (2024-2028)
- A game of hide and seek: targeting neuroblastoma’s escape from immunotherapy – Horizon Europe MSCA Cofund Máxima Butterfly and Villa Joep (2023-2027) - News article
- Targeting aberrant ATRX to identify new treatment options for Neuroblastoma - Horizon Europe MSCA Cofund Máxima Butterfly (2023-2027) - News article
- Joining forces to activate T cell immunity against High-Risk Neuroblastoma (with Nierkens group and van Heesch group) – Villa Joep (2021-2025) - News article
- Targetable immunoregulatory mechanisms in pediatric tumors – Roche (2023-2025)
- Strategic optimalization of the clinical implementation of BCL-2 targeted compounds for neuroblastoma treatment - Villa Joep (2023)
- Validation of Actionable Genomic Abberations in a Pediatric Oncology Network for Doctorate Students (VAGABOND) (with Drost group and den Boer group) - H2020 MSCA ITN (2020-2024) - News article
- Informing treatment decisions of relapsed neuroblastomas by inference of tumor evolution (INFER-NB) - EraCoSysMed (2020-2023)
- Individualized Pediatric Cure (iPC): Cloud-based virtual-patient models for precision pediatric oncology – H2020 SC1-DTH (2019-2023)
- Clinical implementation of Multidimensional Phenotypical drug sensitivities in pediatric precision oncology (COMPASS) – EraPerMed (2019-2023)
- Release the beast: Boosting CAR‐T cell immunotherapy for neuroblastoma - Veni (2022-2025) - News article
- ITCC Pediatric Preclinical POC Platform (ITCC-P4) (with Kool group, Heidenreich and other groups Máxima) – IMI2 H2020 (2018-2023)
- Clinical implementation of a pediatric cancer precision medicine program, enforced with personalized models (iTHER)- ZonMW (2018-2023) - News article
- Precision medicine drug combination testing in neuroblastoma organoids to guide clinical trials (PREDICT) – ERC Starting Grant (2017-2022)
- Combining targeted compounds in neuroblastoma tumors; is two better than one? VIDI ZonMW (2015-2020)
Twinning program KiTZ
Development and implementation of a personalized drug sensitivity profiling pipeline as stratification method for pediatric patients with solid and CNS tumors (2022-2023) - News article
Integrative analysis of neuroblastoma by single-cell RNA sequencing identifies the NECTIN2-TIGIT axis as a target for immunotherapy. J Wienke,L Visser, W Kholosy, K Keller,M Barisa,E Poon,S Munnings-Tomes,C Himsworth, E Calton, A Rodriguez, R Bernardi,F van den Ham, S van Hooff, Y Matser, M Tas, K Langenberg, P Lijnzaad, A Borst, E Zappa, F Bergsma, J Strijker, B Verhoeven, S Mei, A Kramdi, R Restuadi, A Sanchez-Bernabeu, A Cornel, F Holstege, J Gray, G Tytgat, M Scheijde-Vermeulen, M Wijnen, M Dierselhuis, K Straathof, S Behjati, W Wu, A Heck, J Koster, S Nierkens, I Janoueix-Lerosey, R de Krijger, N Baryawno, L Chesler, J Anderson, H Caron, T Margaritis, M van Noesel and J Molenaar, Cancer Cell 42, 283–300, doi:10.1016/j.ccell.2023.12.008 Pubmed PMID: 38181797
Implementation of paediatric precision oncology into clinical practice: The Individualized Therapies for Children with cancer program 'iTHER'. K Langenberg, M Meister, J Bakhuizen, J Boer, N van Eijkelenburg, E Hulleman, U Ilan, E Looze, M Dierselhuis, J van der Lugt, W Breunis, L Schild, K Ober, S van Hooff, M Scheijde-Vermeulen, L Hiemcke-Jiwa, U Flucke, M Kranendonk, P Wesseling, E Sonneveld, S Punt, A Boltjes, F van Dijk, E Verwiel, R Volckmann, J Hehir-Kwa, L Kester, M Koudijs, E Waanders, F Holstege, J Vormoor, E Hoving, M van Noesel, R Pieters, M Kool, M Stumpf, M Blattner-Johnson, G Balasubramanian, C Van Tilburg, B Jones, D Jones, O Witt, S Pfister, M Jongmans, R Kuiper, R de Krijger, M Wijnen, M den Boer, M Zwaan, P Kemmeren, J Koster, B Tops, B Goemans, J Molenaar. Eur J Cancer. 2022 Nov:175:311-325. doi: 10.1016/j.ejca.2022.09.001 Pubmed ID 36182817
Tumor to normal single-cell mRNA comparisons reveal a pan-neuroblastoma cancer cell. Kildisiute G, Kholosy WM, Young MD, Roberts K, Elmentaite R, van Hooff SR, Pacyna CN, Khabirova E, Piapi A, Thevanesan C, Bugallo-Blanco E, Burke C, Mamanova L, Keller KM, Langenberg-Ververgaert KPS, Lijnzaad P, Margaritis T, Holstege FCP, Tas ML, Wijnen MHWA, van Noesel MM, Del Valle I, Barone G, van der Linden R, Duncan C, Anderson J, Achermann JC, Haniffa M, Teichmann SA, Rampling D, Sebire NJ, He X, de Krijger RR, Barker RA, Meyer KB, Bayraktar O, Straathof K, Molenaar JJ (corresponding author), Behjati S. Science Advances. 2021 Feb 5;7(6):eabd3311. doi: 10.1126/sciadv.abd3311. Pubmed PMID: 33547074
Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Eleveld TF, Oldridge DA, V Bernard, J Koster, LC Daage, SJ Diskin, L Schild, N Bentahar, A Bellini, M Chicard, E Lapouble, V Combaret, P Legoix-Né, J Michon, TJ Pugh, LS Hart, JA Rader, EF Attiyeh, JS Wei, S Zhang, A Naranjo, JM Gastier-Foster, MD Hogarty, MA. Smith, JM Guidry Auvil. TBK Watkins, DA Zwijnenburg, ME Ebus, P v Sluis, A Hakkert, E v Wezel, CE vd Schoot, EM Westerhout, JH Schulte, GA Tytgat, MEM Dolman, , I Janoueix-Lerosey, DS Gerhard, HN Caron, O Delattre, J Khan, R Versteeg, G Schleiermacher, Molenaar JJ (corr. author), Maris JM. Nature Genetics. 2015 (IF 29,4) Pubmed PMID: 26121087
Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, Hamdi M, van Nes J, Westerman BA, van Arkel J, Ebus ME, Haneveld F, Lakeman A, Schild L, Molenaar P, Stroeken P, van Noesel MM, Ora I, Santo EE, Caron HN, Westerhout EM, Versteeg R. Nature. 2012 (IF:41,5) Feb 22;483(7391):589-93. Pubmed PMID: 22367537


























