Clinical research is essential to achieve the mission of the Princess Máxima Center for pediatric oncology: to cure every child with cancer, with optimal quality of life. The Trial and Data Center (TDC) facilitates high-quality clinical research with optimal support of knowledge and data, and broad service and expertise.
More than 100 clinical studies are ongoing at the Máxima center, and that number is constantly increasing. As the national center for pediatric oncology, the Máxima center coordinates all Dutch studies in the field of pediatric oncology. These include registration studies for new drugs of interest for pediatric oncology, such as brigatinib and venetoclax.
In addition, the Máxima center is more and more often acting as a sponsor of international studies. For example, Interfant-21 and CHIP-AML. Due to patient access and international collaborations (among others ITCC, Pedal, NANT, SIOPe and PNOC consortia), the Máxima center has a strong international position. There is no other comparable sponsor within the European Union.
The TDC is the central core facility (one-stop shop) for all clinical studies and data requests at the Máxima center.
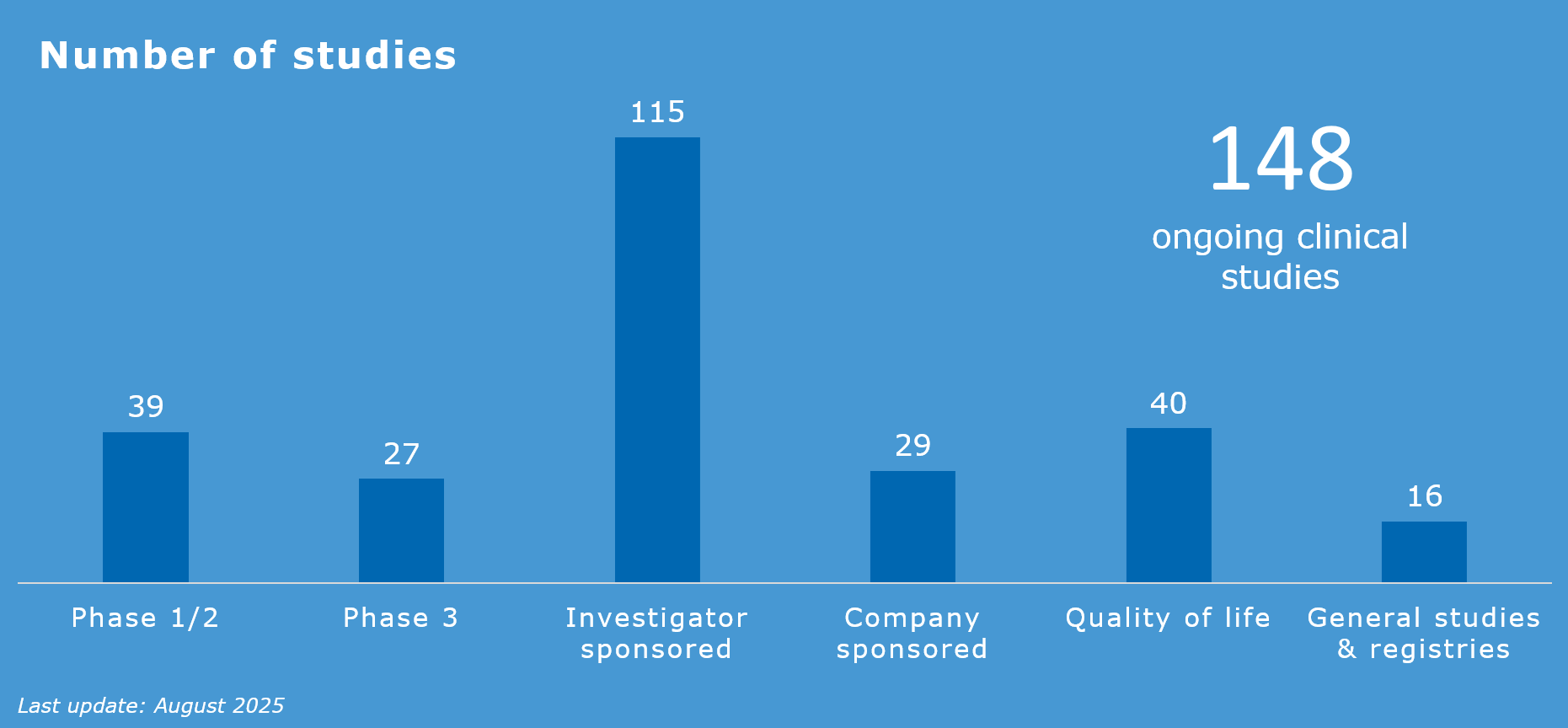
Clinical studies
The TDC handles the implementation and execution of all clinical studies for which patients are being recruited at the Máxima center. This includes studies initiated by researchers in academic centers as well as studies initiated by pharmaceutical companies. In addition, the TDC provides design and setup of investigator-initiated studies conducted both nationally and internationally.
This includes:
- All phases of clinical trials with medicinal products (with a focus on phase 1/2 studies)
- Studies focused on new treatments such as new types of radiotherapy and new surgical methods
- Studies on quality of life, including, for example, studies on long-term effects (LATER), supportive care and psycho-oncology
For this, the TDC provides:
- Support in designing the study and writing the protocol
- Budgeting and arranging contracts
- Guidance in all procedures surrounding study submission
- Design and development of databases
- Responsible for handling patient safety issues
- Registration of all pediatric tumors in the Dutch Cancer Registry and in specific (international) tumor registries
Data provisioning
The TDC is responsible for the processing, delivery and interpretation of clinical data for the purpose of research and quality. The TDC provides support and advice on the use of clinical data and related business operations. This concerns, among others, study design and execution of studies, registries and protocols, research data governance, data quality and integrity, and innovative future (research) data facilities. Our services include:
- Design, development and maintenance of Case Report Forms (CRFs) and corresponding databases
- Export and management of databases, among which the Dutch Childhood Cancer Registry (NKKR)
- Validation and quality of registrations
- Guideline development and evaluation, in collaboration with SKION
- Design and visualization of data solutions such as reports or dashboards
- Statistical analyses and development of conceptual, logical and statistical data models in the field of pediatric oncology
- Data warehouse storage and curation
- National and international data sharing, retrieving and storing of enriched data
- Mapping data to and management of (inter)national standards, e.g.: ICD-O, ICD-10/ICD-11, openEHR, SNOMED-CT, CDISC
- Acting as the Internal Trusted Party within the entire clinical research department
- Data science applications, such as predictive algorithms and models, machine learning, and artificial intelligence
High quality service
With some 100 motivated and enthusiastic employees, the TDC features broad expertise with which we strive to provide efficient and high-quality service. All employees undergo continuous training and much attention is paid to innovation.
The TDC works according to international standards (Good Clinical Practice) and regarding data according to the principles of FAIR (Findable, Accessible, Interoperable, Reusable). With our own quality management system and standard operating procedures (SOPs), we ensure quality. We play an active role in the integration of research and care and cooperate with international partners.
Setting up a clinical trial or submitting a data request?
All research proposals involving patients of the Máxima center, their data and/or materials are reviewed by the Scientific Committee (SciCom), consisting of the Clinical Research Committee (CRC) and the Biobank and Data Access Committee (BDAC).
- Scientists from external institutes may submit a research proposal in collaboration with a research group or clinical scientist of the Máxima center. Please contact one of our research groups to discuss the research proposal, or contact the SciCom for more information.(scicom@prinsesmaximacentrum.nl)
- Máxima researchers: More information on the internal review procedures can be found here.
- Before a research proposal is reviewed by the CRC/BDAC, an intake takes place to outline commitment of human and financial resources.
Who we are:
The TDC is led by Prof. Dr. Michel Zwaan (pediatric oncologist) and Dr. Harm van Tinteren (statistician), supported by business operations manager Steven Vanhoutvin and team leaders Edith Schasfoort (site), Karolien Makkink (trial management) and Rinke Riezebos (data management).
Site
The site team of the TDC handles the implementation of all studies for which patients are being recruited in the Máxima center. This concerns both studies initiated by investigators in academic centers as well as studies set up by pharmaceutical companies.
Trial support/Start-up coordination:
- ‘Spider in the web’ in the local implementation and conduct of clinical studies
- Coordinates start-up of new studies
- First point of contact for researchers, sponsors/pharmaceutical companies and other involved parties
Research nurses/coordinators/assistants:
- Perform patient-related tasks regarding the various study protocols, including patient information
- Collect data and take care of administration concerning the study
- Perform ECGs for studies and in care
- Coordinate Biobank PIF
Local data management:
- Data collection and registration in a Case Report Form (CRF)
- Registration and randomisation of patients, checking in- and exclusion criteria
- Registration of all pediatric tumors in the Dutch Cancer Registry and in specific (international) tumor registries
Team leader: Edith Schasfoort
Trial management
Trial management is concerned with the set-up and coordination of investigator-initiated studies conducted both locally and internationally by participating centers around the world. Trial managers work closely with investigators and provide end-to-end process management of clinical trials, supported by clinical trial assistants.
Depending on the type of study, trial management is responsible for:
Before start:
- Intakes of new research proposals for discussion and approval by the CRC
- Support in protocol development and writing of patient information
- Guidance on the submission procedure for the ethical committee and competent authorities and the resulting requirements during the course of the study
- Responsible for project planning, assisting in preparation of study budgets, document management, and preparation of reports
- Together with KTO, contracting participating centers, laboratories and other vendors for such things as medication distribution and monitoring
- Preparing procedure manuals for laboratories and pharmacy
During the study:
- Safety Desk: reporting, processing or advising on safety reports (SAEs, SUSARs, DSURs) to authorities
- Managing vendors and study-specific teams, contact with participating centers
- Supervising PhD students
- Arranging and supervising medication distribution to participating centers
- Facilitating payment of invoices
- Closing and archiving of research
Team leader: Karolien Makkink
Clinical data provisioning
Within the TDC, the Clinical Data Provisioning team is responsible for the processing and delivery of data for the purpose of research and quality.
The team consists of several sub-teams, each with its own task:
Central data management:
- Design, development and maintenance of CRFs and corresponding databases for clinical trials
- Supervising PhD students
Clinical Data Analysis and Advisory Group (CDAAG):
- Export and management of databases, among which the Dutch Childhood Cancer Registry (NKKR)
- Validation and quality of registrations
- Guideline development and evaluation, in collaboration with SKION
- Design and visualization of data solutions such as reports or dashboards
- Statistical analyses and development of conceptual, logical and statistical data models in the field of pediatric oncology
- Data warehouse storage and curation
- National and international data sharing, retrieving and storing of enriched data
- Mapping data to and management of (inter)national standards, e.g.: ICD-O, ICD-10/ICD-11, openEHR, SNOMED-CT, CDISC
- Acting as the Internal Trusted Party within the entire clinical research department
- Data science applications, such as predictive algorithms and models, machine learning, and artificial intelligence
- Within the Máxima center, CDAAG collaborates with IDT-Data Intelligence and Big Data Core
Statisticians:
Provide support in all phases of prospective clinical research, from design to final analysis
Team leader: Rinke Riezebos
Quality
All clinical research is to be conducted in accordance with applicable laws and regulations, including ‘Good Clinical Practice’ (GCP). This is audited on a regular basis. The quality officers are responsible for the quality management system of the department. They also develop and teach the center-specific meeting of the BROK® course (Basic Course in Regulation and Organization for Clinical Researchers) for all staff involved in clinical research.
Quality officers: Angela Vlug, Marlien Beusink and Esther Caspers
Business operations
The Business Operations team is responsible for budgeting studies and drafting and completing all study-related contracts. They make arrangements and maintain contracts with pharmaceutical companies and other suppliers (e.g. software companies). For additional legal support, the TDC forwards your question to the Knowledge Transfer Office (KTO).
Business operations manager: Steven Vanhoutvin
Contact: (TDC general)
